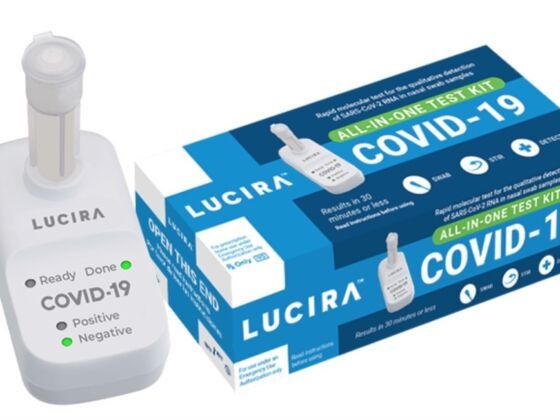
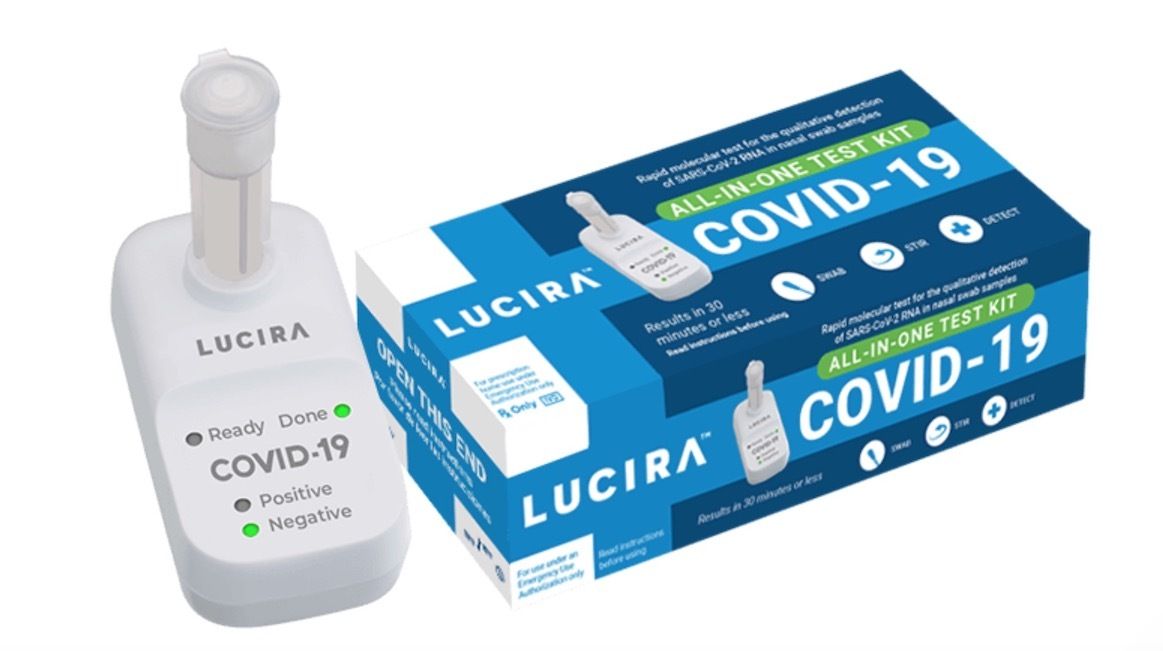
On Tuesday, the FDA approved the first COVID-19 diagnostic at-home self-test that provides rapid results, making it easier for people to quickly determine whether or not they are infected by the novel coronavirus.
The Lucira COVID-19 All-In-One Test Kit is a molecular single-use kit that works by swirling a self-collected swabbed sample in a vial. The sample is then placed in a hand-held test unit and can provide results in 30 minutes or less. A light on the display will show whether the sample tested positive or negative for COVID-19.